Chemical Reactions
Abstract
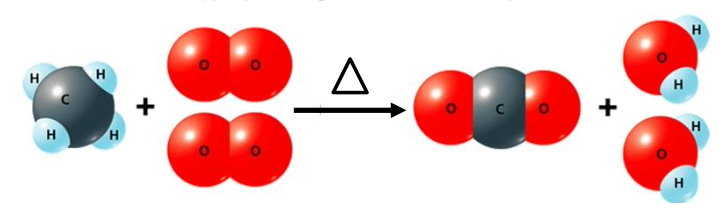
Chemical change involves a reorganization of the atoms in one or more substances. Chemical reactions can be represented in chemical equations using words, structures, or chemical formulas. Since chemical reactions fulfill the Conservation of Mass Law that atoms are neither created nor destroyed during a chemical process, chemical equations must be balanced with the same number of each element before and after the reaction. Chemical reactions can be classified by generalized reaction types.
After completing this activity, students will be able to identify reactants and products in a chemical reaction from different representations (words, formulas, symbols, models); write a balanced chemical equation; and identify the type of chemical reaction from the chemical equation.
Level: Undergraduate
Setting: Classroom
Activity Type: Learning Cycle
Discipline: Chemistry
Course: General Chemistry / Introductory Chemistry
Keywords: Writing, Balancing, & Classifying Chemical Reactions
Downloads
Published
How to Cite
Issue
Section
License
Copyright of this work and the permissions granted to users of the PAC are defined in the PAC Activity User License.

