Reversible Changes of an Ideal Gas
Abstract
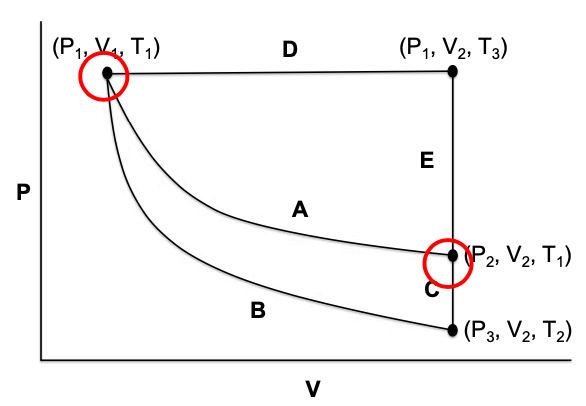
This activity is designed to help students apply thermodynamics equations and definitions to solve for expressions for heat, work, and change in internal energy for an ideal gas undergoing various reversible changes. The model, a pressure-volume diagram without numerical values, aids students in the identification of conditions of a process, whether isobaric, isochoric, isothermal, or adiabatic, as they develop problem solving skills. Along the way students practice using integration while collaborating with peers, which will prove useful as they embark individually on numerical problems outside of class.
Level: Undergraduate
Setting: Classroom
Activity Type: Application
Discipline: Chemistry
Course: Thermodynamics
Keywords: Reversible changes, ideal gas, heat, work, internal energy
Downloads
Published
How to Cite
Issue
Section
License
Copyright of this work and the permissions granted to users of the PAC are defined in the PAC Activity User License.

