What Happens When Compounds are Added to Water?
Abstract
This activity aims to deepen or refine students understanding of the dissolution process. The activity guides students from the macroscopic level to the symbolic and particulate levels. This activity begins with students observing the dissolution of a salt (sodium chloride) and a molecule (methanol) at the macroscopic level. Students are then provided with 3D molecular models to explore dissolving using tactile models to guide in visualizing the abstract particulate level. From there, students are provided conductivity data as additional evidence to refine their skills at writing chemical equations and drawing particulate diagrams to represent what happens when ionic and molecular compounds dissolve in solution.
Level: Undergraduate or Advanced High School
Setting: Classroom
Activity Type: Application
Discipline: Chemistry
Course: General Chemistry
Keywords: solution chemistry, molarity, ionic compounds, molecular compounds, particulate nature of matter, conductivity.
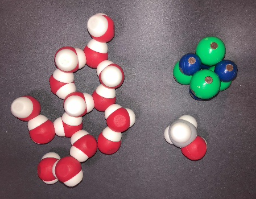
Downloads
Published
How to Cite
Issue
Section
License
Copyright of this work and the permissions granted to users of the PAC are defined in the PAC Activity User License.

