Lewis Acids and Bases: MO Depiction
How can we describe acid/base reactions in terms of electron transfer?
Abstract
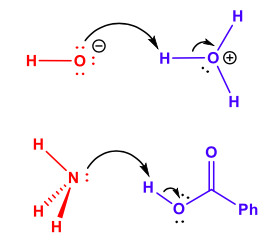
This activity is designed for undergraduate students enrolled in Inorganic Chemistry. Students should have previously taken two semesters of General Chemistry and have Organic Chemistry as a co- or pre-requisite. Topics covered in Inorganic Chemistry prior to this activity should include a review of Lewis Structures and Molecular Orbital Theory. This activity introduces Lewis acid and base concepts and explores them through the lens of molecular orbital theory. After completing this activity, students should be able to identify Lewis acids and bases in reactions but also describe how frontier molecular orbitals differ between reactants and products of a Lewis acid/base reaction. Changes in molecular orbital energies are illustrated through color changes and absorption spectroscopy.
Level: Undergraduate
Setting: Classroom
Activity Type: Learning Cycle
Discipline: Chemistry
Course: Inorganic Chemistry
Keywords: Lewis Acid, Lewis Base, Molecular Orbital, Frontier Orbital
Downloads
Published
How to Cite
Issue
Section
License
Copyright of this work and the permissions granted to users of the PAC are defined in the PAC Activity User License.

