Chemical Combination Laws
Abstract
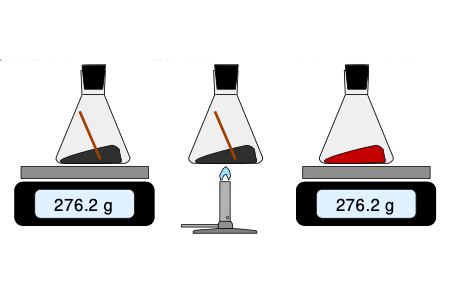
Atoms combine to form compounds, the identity of which is determined by the number and types of atoms it contains and represented by the chemical formula. Early experiments demonstrated that elements combine in particular ratios, which are stated in the Law of Definite Proportions, the Law of Multiple Proportions, and the Law of Conservation of Mass. The chemical combination laws can be used to demonstrate the relationship between mass, moles, and numbers of atoms or molecules represented by the chemical formula.
After completing this activity, students will be able to recite the chemical combination laws; define, interpret, and write a chemical formula; and calculate formula mass for compounds.
Level: Undergraduate
Environment: Classroom
Activity Type: Learning Cycle
Discipline: Chemistry
Course: General Chemistry / Introductory Chemistry
Keywords: Conservation of Mass, Definite Proportions, Multiple Proportions, Chemical Formula, Formula Mass, Mole, Avogadro's Number
Downloads
Published
How to Cite
Issue
Section
License
Copyright of this work and the permissions granted to users of the PAC are defined in the PAC Activity User License.

