Electromagnetic Radiation
Abstract
Purpose:
Light is the visible part of a vast spectrum of electromagnetic radiation (EMR), which is composed of perpendicular electrical and magnetic fields. The characteristics of EMR indicate that light behaves as both a wave and a particle. Visible light and other forms of EMR are essential to our understanding of chemical behavior and structure.
Content Learning Objectives:
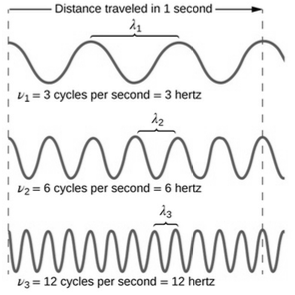
• Describe light as a wave and express the relationships between wavelength, frequency, and the speed of light.
• Describe light as a particle of energy (photon) relating frequency and wavelength to energy.
• Use energy level transition diagrams to correlate absorption and emission of light to the quantized energy levels within an atom.
Process Skill Goal:
• Students will develop their problem solving skills by describing the relationships between properties of light and utilizing those relationships to understand atomic spectra.
Level: High School, Undergraduate
Setting: Classroom
Activity Type: Learning Cycle
Discipline: Chemistry
Course: Introductory Chemistry
Keywords: electromagnetic radiation, wave properties, photon, absorption, emission, energy transitions
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Maury HowardCopyright of this work and the permissions granted to users of the PAC are defined in the PAC Activity User License.

