Evolution of Atomic Theory
Abstract
Purpose:
Greek philosophers Leucippus and Democritus deduced that matter was composed of small particles that were indivisible and that differed in size, shape, and weight. Over 2000 years later, John Dalton provided the experimental evidence that matter was indeed composed of discrete particles. His model represents the atom as a sphere of uniform density, and that each element had atoms of different masses and chemical properties. In the late 1800s and early 1900s, new technology allowed for experimental exploration of elements that lead to discovery of subatomic particles and revised our model of the atom.
Content Learning Objectives:
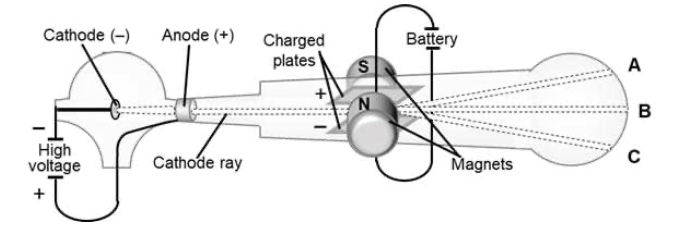
● Students will be able to summarize and interpret the experimental results of Thompson and Rutherford.
● Students will be able to describe how the atomic model has changed as experimental data became available.
● Students will be able to identify and describe the subatomic components of the atom.
Process Skill Goal:
● Students will develop critical thinking skills by analyzing and evaluating relevant information to make predictions and reach conclusions supported with evidence.
Level: High School, Undergraduate
Setting: Classroom
Activity Type: Learning Cycle
Discipline: Chemistry
Course: Introductory Chemistry
Keywords: atomic theory, Rutherford, plum pudding, subatomic particles, gold foil, cathode ray
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Maury HowardCopyright of this work and the permissions granted to users of the PAC are defined in the PAC Activity User License.

