Properties of Acids and Bases
Abstract
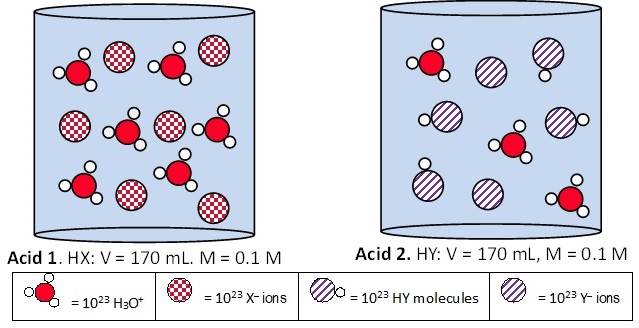
This activity for a one-semester GOB course for health professions, introduces properties of acids and bases. Students explore equations for reactions of acids and bases (weak and strong) with water to identify characteristics of acids, bases and amphoteric compounds. Brønsted-Lowry concepts are introduced through reactions of a generic acid (HA) with water, and base (B:) with water. Students connect the acid and conjugate base using red markers, and the base and conjugate acid with blue. Students explore images of relative amounts of ions (H3O+, X–, Y–) and molecules (HY) to develop explanation for the difference between strong and weak acids/bases.
Learning objectives:
- Identify characteristics common for acids, bases and amphoteric compounds.
- Write balanced equations for reactions of Brønsted-Lowry acids (and Brønsted-Lowry base) with water and identify the conjugates.
- Working as a team identify and explain differences between strong and weak acids and between strong and weak bases.
Level: Undergraduate
Setting: Classroom
Activity Type: Learning Cycle
Discipline: Chemistry
Course: General, Organic & Biochemistry (GOB)
Keywords: Brønsted-Lowry acids and bases, conjugate acid, conjugate base, amphoteric, weak/strong acid, weak/strong base
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Elisabeth Bell-LoncellaCopyright of this work and the permissions granted to users of the PAC are defined in the PAC Activity User License.

